Drug watchdog halts injections after adverse reaction in patients
Adam Cresswell From: The Australian April 19, 2011 12:00AM
THE drug regulator has told GPs to stop giving patients a second dose of a vaccine that protects against pneumococcal disease, after more than 80 Australians suffered severe reactions, including severe swelling and abcesses.
The Therapeutic Goods Administration said it was investigating what could have caused 178 reports of reactions to the Pneumovax 23 vaccine, which is meant to protect against a potentially life-threatening bacterial infection that can cause meningitis and death and is mainly given to adults.
Of the 178 reaction reports made from January 1 to April 14, 169 related to reactions at the injection site, of which 82 were deemed severe, and included the skin inflammation cellulitis, swelling from the shoulder to the elbow and abcesses.
In a statement, the TGA said that although such reactions were specifically mentioned as possible side-effects in the information provided with the vaccine, the sheer number had triggered the agency's concern.
The total number of reaction reports is nearly three times the 63 adverse reactions that were reported to the end of April last year, and more than five times the 34 reactions reported in 2009.
The vaccine, made by Merck Sharp and Dohme, is provided free once every five years for adults over 65, indigenous people over 50, tobacco smokers, and people aged 10 and over who are predisposed to invasive pneumococcal disease.
The latest scare follows an earlier incident with the same vaccine in March, when the TGA ordered a recall of one specific batch after a cluster of seven patients reported similar reactions.
Of the 178 reaction reports overall, 57 were among people who received the suspect N3336 batch involved in the earlier NSW incident.
The TGA said in a statement that, out of the 82 severe reactions, 44 were among people receiving their second dose. Another 22 were in people receiving the vaccine for the first time, while it was unclear whether the remainder were first or second shots.
"Analysis of these adverse reactions suggests that the largest number of reactions is occurring in people receiving their second five-yearly dose of Pneumovax," the statement said.
"Further detailed analysis . . . is required and will be undertaken by the TGA and the Australian Technical Advisory Group on Immunisation. Until this analysis is complete, the TGA is recommending as a precautionary measure that patients do not receive a second dose."
Merck Sharp and Dohme said the company was not involved in the TGA's alert and could not "elaborate on the reasons" for the warning.
News and commentary on the autism epidemic and my beautiful boy who is living with autism.
April 22, 2011
Merck's Pnumovax Pulled in Australia with 82 Severe Reactions Since January
October 11, 2010
Thank You Mr. Gadsden
“I want to sell drugs to everyone. I want to sell drugs to healthy people. I want drugs to sell like chewing gum.” With those words former Merck CEO Henry Gadsden established the aim of the entire pharmaceutical industry more than 30 years ago.
- Diary of a Legal Drug Dealer
Do you get that they don't care if our kids are over vaccinated? Do you get that?
August 14, 2010
Did Anyone Know that the CEO of Reuters was on the Board of Merck?
Wonder if it skews Reuters coverage of let's say... MMR or Gardasil or RotaTeq or....
Thomas H. Glocer
Chief executive officer, Thomson Reuters Corporation (information and services company for businesses and professionals). Director, Thomson Reuters Corporation, Partnership for New York City. New Merck director since November 3, 2009
No mention here that the CEO of the news agency reporting had a very vested interest in seeing MMR exonerated, now is there. And Dr. Wakefield said the charges were unjust... did they look into his claim?
British ban for doctor at heart of MMR vaccine row
By Kate Kelland, Health and Science Correspondent
LONDON | Mon May 24, 2010 12:44pm BST
LONDON (Reuters) - A doctor whose claims of links between vaccination and autism triggered a scientific storm before being widely discredited was struck off the medical register Monday for professional misconduct.
Dr Andrew Wakefield's 1998 study led many parents to refuse to have their children vaccinated with the measles, mumps and rubella (MMR) shot and has been blamed for a big rise in measles cases in the United States and parts of Europe in recent years.
A disciplinary panel of the General Medical Council (GMC) found that Wakefield had acted in a "dishonest," "misleading" and "irresponsible" way during his research.
The ruling means Wakefield, who now lives and works in the United States, can no longer practise as a doctor in Britain, but can continue to work in medicine outside the UK.
His paper, published in The Lancet medical journal but since widely discredited, caused one of the biggest medical rows in a generation.
"The panel has determined that Dr Wakefield's name should be erased from the medical register," the GMC said in a statement.
Wakefield had failed to disclose various details about the funding of the study -- a failure the GMC described as "dishonest and misleading" -- and had acted "contrary to the clinical interests" of the children involved in his research.
Striking Wakefield off the medical register was "the only sanction that is appropriate to protect patients" and was in the wider public interest. It was also "proportionate to the serious and wide-ranging findings made against him," the statement said.
Data released last February for England and Wales showed a rise in measles cases of more than 70 percent in 2008 from the previous year, mostly due to a fall in the number of children being vaccinated. Vaccination rates are now recovering.
Terence Stephenson, president of the Royal College of Paediatrics and Child Health, said the false suggestion of a link between autism and the MMR vaccine had caused "untold damage" to vaccination programs.
"We cannot stress too strongly that all children and young people should have the MMR vaccine. Overwhelming scientific evidence shows that it is safe," he said in a statement.
Wakefield defended his work, and said the GMC had sought to deny that the case against him was related to whether the vaccine was safe, and specifically, whether it caused autism.
"Efforts to discredit and silence me through the GMC process have provided a screen to shield the government from exposure on the ... MMR vaccine scandal," he said in a statement.
The GMC said his refusal to accept that he had made mistakes meant that a temporary suspension of Wakefield's licence was not enough and he should be banned altogether.
"Dr Wakefield's continued lack of insight as to his misconduct serve only to satisfy the panel that suspension is not sufficient and that his actions are incompatible with his continued registration as a medical practitioner," it said.
(Editing by Andrew Roche)
June 13, 2010
Why were Merck's Lawyers at the Cedillo Appeal Last Week
What is Merck's interest here? (duh.) Is Merck helping out the DOJ in their defense? If so, what help are they offering?
And what of the rumors of Pharma payouts to families in the vaccine court? It is legend that on occasion, when a family has a slam dunk case, the pharmaceutical company that made the vaccine in question steps in privately, offers the family a shocking amount of money, and the case is quietly withdrawn from the VICP. Non-disclosures of course would be iron clad.
This of course protects companies like, let's say Merck, from having their product linked publicly to autism in a court ruling, and prevents precedent from being set.
Was Merck in DC to help out the DOJ? Or are they worried that this will get remanded back to the Special Master with instructions that read: "Do it again, this time with out corruption" and Michelle will win the case, so they are trying to figure out how big a check it would take to keep the Cedillos happy and quiet?
So... to the DOJ... are Merck's lawyers assisting you on this case?
And Merck (or any pharma willing to speak out) have you been paying families to leave the vaccine court to cover your billion dollar assets?
This is supposed to be a non-adversarial process. Is there anything on the planet more adversarial than a Pharma lawyer?
February 12, 2010
Anatomy of a Witch Hunt
Also fascinating that they have tried to portray Wakefield as the guy that invented the autism/vaccine connection, despite the fact that Leo Kanner reported that one of his first 11 cases in the 1940's was a regression following a smallpox vaccine, the VCIP has been paying autism cases for 25 years, and I first heard about the connection in my undergrad psych program in 1988 at George Mason University, so that they can use this GMC hearing to declare the vaccine controversy over. (I have forty or so studies on my "no evidence of any link" page supporting the vaccine/autism connection and I have never even had Wakefield's MMR paper up there.
Fortunately AOA has been all over this.
Today I had an hour and started mapping out the conflicts on interests in all of the forces that are posing as unbiased sources and trashing Wakefield and his work by charging him with conflicts of interest. The irony is lost on too many people.
It is a work in progress. One of my friends in the UK is looking it over to help fill in more of the blanks, so it will be updated.
A few notes not on the chart. Judge Nigel Davis is the judge who ruled that families trying to sue GlaxoSmithKline for the damage done to their children by the MMR would not be given legal aid to do so, ending MMR litigation in the UK. His brother is Sir Crispin Davis, who was the CEO of Elsevier, publisher of The Lancet and was also on the board of GlaxoSmithKline. Additionally... Paul Offit is an industry spokes person for Merck, that was too long to fit into the chart, so I used the more pejorative, "lap dog". And there is word out this afternoon that another Elsevier journal may be trying to bury the Hep B monkey study that Wakefield worked on, although no word from the journal on this yet.
Look at the energy flow in this thing... Props to Dr. Wakefield and his compadres for not backing down under this insane amount of industrial pressure. I mean just look at this billion dollar medical/pharma/media/(arms sales?) unprincipled conglomco machine! Eliot Ness wasn't even up against this big of a beast when he took on the mob. And I have not even included any of the public health infrastructure, or the GMC in this flow chart.
Keep your head up England, and understand that you are under a blitz. They are now officially throwing everything they have at you and they are only exposing their own corruption. Never, never, never, never give up.
Problem for the beast is... they are using all their ammo, and six months from now, nothing will have changed for them. Because the public knows they are full of crap.
click to see it full size.
UPDATE:
A friend in the UK brought something interesting to my attention. It is a flow chart done by a journalist in 1994 on how money and influence flowed around the Burroughs Wellcome pharmaceutical company and all it's satellites (foundations, trusts, doctors, medical institutions, universities, medical journals and even regulatory agencies). One of which is the Wellcome Trust.
I invite to you take a look at the amazingly, unbiased and completely free of conflicts of interesty type research that Wellcome is funding, by reading my piece on Professor Alan Emond's study that shows that kids with autism don't have bowel problems. He claims no conflicts of interest, but fails to mention that he is on the UK's Joint Committee on Vaccination and Immunisation (Britian's version of the CDC's Advisory Committee on Immunization Practices.)
The chart certainly enhances the understanding of how the machine works, but the two facts that make this flow chart so much more interesting is that Burroughs Wellcome of course became Glaxo Wellcome, which became GlaxoSmithKline, who makes the MMR; and that the journalist that created this chart was Brian Deer.
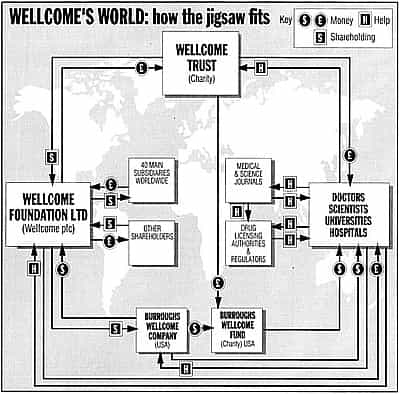
UPDATE: One commenter was looking for a source for my 20 billion dollar figure.
Drugmakers, Doctors Rake in Billions Battling H1N1 Flu
Swine Flu Is Bad for Victims, But Good for Businesses That Cater to Expanding Market
By DALIA FAHMY
Oct. 14, 2009
ABC News/Money
Americans are still debating whether to roll up their sleeves for a swine flu shot, but companies have already figured it out: vaccines are good for business.
h1n1.
Drug companies have sold $1.5 billion worth of swine flu shots, in addition to the $1 billion for seasonal flu they booked earlier this year. These inoculations are part of a much wider and rapidly growing $20 billion global vaccine market.
"The vaccine market is booming," says Bruce Carlson, spokesperson at market research firm Kalorama, which publishes an annual survey of the vaccine industry. "It's an enormous growth area for pharmaceuticals at a time when other areas are not doing so well," he says, noting that the pipeline for more traditional blockbuster drugs such as Lipitor and Nexium has thinned.
As always with pandemic flus, taxpayers are footing the $1.5 billion check for the 250 million swine flu vaccines that the government has ordered so far and will be distributing free to doctors, pharmacies and schools. In addition, Congress has set aside more than $10 billion this year to research flu viruses, monitor H1N1's progress and educate the public about prevention.
Drugmakers pocket most of the revenues from flu sales, with Sanofi-Pasteur, Glaxo Smith Kline and Novartis cornering most of the market.
But some say it's not just drugmakers who stand to benefit. Doctors collect copayments for special office visits to inject shots, and there have been assertions that these doctors actually profit handsomely from these vaccinations.
It is a notion that Dr. Lori Heim, president of the American Academy of Family Practitioners, says is simply not true.
"According to most of the physicians I have talked to, the administration of these vaccines is done for the community's benefit as opposed to anything that helps profit," she says. Heim adds that even though doctors will not have to shell out for the H1N1 vaccine, they will bear the usual costs associated with storage and administering the shots.
"There is an administration fee, for the costs that you can't get reimbursed through Medicare or Medicaid," she says. "This is usually less than, or right at the break-even point."
Still, pharmacies also charge co-payments or full price of about $25 to those without insurance and often make more money if patients end up shopping for other goods.
"Flu shots present a good opportunity to bring new customers into our stores," says Cassie Richardson, spokesperson for SUPERVALU, one of the country's largest supermarket chains. Drawing customers to the back of a store, where pharmacies are often located, offers retailers a chance to pitch products that might otherwise go unnoticed.
Even companies outside of the medical industry are benefiting: the UPS division that delivers vaccines in specially designed containers, for example, has seen a bump in business.
New Entrants in Flu Shot Business
The intensifying competition has irked some doctors.
"Retailers and other non-medical professionals have siphoned off the passive income that once helped to cover medical overhead," says Dr. Caroline Abruzese, an internist in Atlanta. "The larger retail chains can invest up front in large volumes of vaccine at low prices, and market to customers already in their stores."
The promise of profits has attracted new players into the business. Some of the world's largest drugmakers, who in the past avoided the vaccine market because of its limited scope -- its not easy to convince healthy adults to get a shot for measles -- are now jumping into the fray.
Last month alone saw three large vaccine deals. Abbott Labs bought a Belgian drug business, along with its flu vaccine facilities, for $6.6 billion. Johnson & Johnson invested $444 million in a Dutch biotech firm that makes and develops flu vaccines. Merck, which already makes vaccines for shingles and other diseases, struck a deal to distribute flu shots made by Australian CSL.
Smaller biotechs are also angling for a slice of the action, making vaccines one of the fastest-growing areas of research in the biotech industry.
Large and small drugmakers are drawn to the business largely because of scientific advances that promise to radically expand the range of health problems that vaccines can address. In addition to preventing childhood diseases such as measles and polio, vaccines can now also ward off cervical cancer, and researchers are working on vaccines for HIV and tuberculosis.
Scientists believe they can create therapeutic vaccines than treat diseases such as Alzheimers and diabetes after they have set in. (At least one company is betting on a vaccine that helps cigarette smokers quit.)
"These innovations broaden the market potential for vaccine makers and partly explained the renewed interest by drugmakers," says Anthony Cox, a professor at Indiana University's Kelley School of Business who specializes in the marketing of medical products.
But Mark Grayson, a spokesperson for the Pharmaceutical Research and Manufacturers of America, which represents the country's leading pharmaceutical research and biotechnology companies, says that drugmakers are also compelled by the government to join efforts to ensure that there is enough vaccine to go around.
"Because of national security implications, the government felt that they needed to encourage and ask [vaccine manufacturers] to move much quicker," he says. Grayson adds that vaccine manufacturers also face significant costs; aside from the expense of fitting a new vaccine into a tight production schedule, drugmakers GlaxoSmithKline and Sanofi Pasteur were forced to acquire new vaccine production facilities in recent years to keep up with demand.
Alternatives to Vaccines Are Few
While this promise of new treatments for painful diseases brings hope to many, vaccines continue to attract critics. The National Vaccine Information Center, a non-profit advocacy group, is at the forefront of a movement demanding that vaccines be tested more thoroughly before hitting the market. Although there has been little evidence to support their claim, detractors -- including the comedian Jim Carrey -- believe that vaccines are at least partly to blame for the sharp rise in autism in recent decades.
The swine flu vaccine has also attracted its share of critics. Frank Lipman, a New York-based doctor who specializes in a mix of Western and alternative medicine, points out that the swine flu is rarely fatal and that it's too early to tell if it's safe because it hasn't been widely tested.
Others argue that Americans have little choice. The cost of a widespread pandemic, similar to Spanish Flu outbreak in 1918, which killed 675,000 Americans (and 50 million worldwide), would be devastating. The Trust for America's Health, a Washington-based non-profit organization, estimates that a severe pandemic could push down GDP by more than 5 percent and cost Americans $683 billion.
"We're not seeing a pandemic that's this severe," says Jeff Levi, director of Trust for Americas Health. "We've dodged a lot of bullets."
December 21, 2009
Julie Gerberding Named Head of Merck Vaccines
Wow... Congratulations Julie! Who would have guessed that you would be the one person in the whole world that Merck would want to head their vaccine division?
Oh... that is right... we did.
Me, January 10, 2009:
"Anyone wanna start bets on where our friend Julie will land? Eli Lilly? Merck perhaps? "
Julie's years of "looking and looking" for a vaccine/autism connection with the firm resolve of OJ Simpson looking for the real killers, asinine statements on autism in the press and denialism of an epidemic happening in front of everyone's eyes has finally paid off big for her.
Here is what we do know now... that the head of vaccines for Merck is a liar. And one who tells absurd lies and thinks that people will swallow them. In the age of vaccine skepticism, Merck was so concerned with winning the public trust that they hired the woman who was the at the helm of the autism epidemic for the last eight years.
Brilliant.
But I am guessing that deal with the devil was made long ago.
There is no one whose actions I have had more contempt for in this health disaster than Julie Gerberding. My son regressed into autism following his vaccines on her watch. I became increasingly incensed by her actions, non-actions and thinly veiled vaccine salesmanship as the years rolled on; but I don't think down deep that even I actually believed that out of all the positions in the world she would take THE job with THE company that would truly prove beyond a shadow of a doubt that she was working for PHARMA at the expense of our kids since Bush appointed her as CDC head. If for no other reason out of embarrassment for what it would betray about her.
The woman has no shame.
The position is announced Christmas week, I guess hoping that mom's like me will be too busy trying to keep their kids with autism from eating the glass balls off the yule tree to notice. And it may be that she was legally prevented from taking the job any sooner. Email from someone who travels in these circles:
"...but based on a conversation I recently had with a friend who is a former Bush Administration official, this is pretty close to the minimum interval for how former feds can go to work with a company they regulated after leaving government. January 25th: a year and three business days after Obama’s inauguration (January 20th, 2009). January 25th 2010 is the first Monday she could start".
So as Julie ascends to this esteemed position, lets take a walk down memory lane of her disastrous reign as the chief of our nations public health services at all the bull shit she has had to offer us.
First, a critical review of the only thimerosal/autism study done under Julie's tenure:
Here's Why the Disdain...
And Julie's eventual secret disavowal of the research as junk to Congress while CDC still listing it publicly as research that disproves a vaccine autism connection:
Julie Gerberding Tells Congress That The Verstraeten Study is Junk!
Next, as sampling of her brilliant track record and descent into mockable absurdity:
The Age of Autism: CDC Probes Vaccines
AJC: Autism Controversy Eats at Credibility of CDC
Time: Troubles at the CDC
Julie Gerberding: Getting out the BIG shovel
Another Call for Julie Gerberding's Resignation
CDC: Out of Excuses on the Autism Study that "Should be Done"
Julie Gerberding is Doing an Awsome Job
It Is Time for Julie Gerberding to Step Down
Jenny McCarthy Calls For Julie Gerberding's Resignation
An Invitation to Julie Gerberding to Help Her Find the Missing Information on Autism
Wait! Did Julie Gerberding Just Admit that Vaccines Trigger Autism!?
Our Julie in her Finest Hour trying to convince us that vaccines do and do not cause autism:
Julie Gerberding Admits on CNN that Vaccines can Trigger Autism
Julie digging the hole deeper:
Hey Dr. Gerberding.. What is this "Autism-Like Syndrome"
Gerberding Axes 9/11 Health Official
This is one of my favorites... a video of Julie NOT lying. I was so used to her rehearsed, condescending autism denials that when I saw her talking normally about something that was actually true, that smoking is bad for you, I was a little shocked at the stark difference in her demeanor.
Watch Julie Gerberding Not Lie
The relief when she finally went away:
Julie Gerberding Has Resigned
Julie's sly return while working for PHARMA but pretending that she was still a public health official:
Julie Gerberding Now Officially a Paid Pharma Shill Withholding her Conflicts of Interest
Confirmation that she was acting as a swine flu salesman as a part of the "Edelman Global Task Force on H1N1 Influenza":
Of vaccines, mercury, autism and Julie Gerberding
And finally, her assention to the fruit of her labor selling out the children of the world:
WSJ: Merck Appoints Former CDC Chief As Head Of Vaccines
DOW JONES NEWSWIRES
Merck & Co. (MRK) named former public-health authority Julie Gerberding as the head of the company's $5 billion vaccines business, starting Jan. 25.
Gerberding was the director of the U.S. Centers for Disease Control and Prevention through most of President George W. Bush's administration, from 2002 until earlier this year.
Vaccines, along with emerging markets and biologics, are an area Merck is eyeing for growth as it digests its recent acquisition of Schering-Plough Corp. The takeover was designed to help bolster Merck's research pipeline and diversify its product lineup in the face of challenges such as generic competition and setbacks in bringing new drugs to market.
Merck's previous head of vaccines, Margaret McGlynn, recently retired.
The appointment of Gerberding brings Merck a step closer to rounding out its post-Schering senior leadership team, most of which had been announced in late August but which still had a few holes. Last week, the company named a chief medical officer, Michael Rosenblatt, who had been dean of Tufts University's medical school.
Also, Merck earlier this month named Michael Kamarck as president of Merck BioVentures and senior vice president of vaccines and biologics manufacturing, said spokeswoman Amy Rose. Merck BioVentures is the unit Merck created last year to develop so-called follow-on biologics, or new versions of drugs derived from living cells.
Kamarck was previously head of technical operations and product supply at Wyeth, which was acquired by Pfizer Inc. (PFE). Frank Clyburn, who previously led Merck BioVentures, has been named senior vice president of global pharmaceuticals franchises.
Merck continues to search for a permanent head of its consumer health-care unit, a business largely inherited via the Schering deal, and which sells Coppertone sunscreen among other products. Stanley Barshay is interim leader of the business.
Merck Chairman and Chief Executive Richard Clark on Monday called Gerberding a "preeminent authority in public health, infectious diseases and vaccines."
As president of the vaccines business, she will be responsible for the sale of the company's current portfolio of vaccines, the introduction of vaccines from the company's pipeline and the acceleration of Merck's efforts to broaden vaccinations in developing countries. Gerberding will also collaborate with Merck's manufacturing division and Merck Research Laboratories to manage links between basic research, late-stage development and manufacturing.
Merck shares were recently up 2.25% at $38.24 amid a broad market rally.
-By Joan E. Solsman, Dow Jones Newswires; 212-416-2291; joan.solsman@dowjones.com
(Peter Loftus in Philadelphia contributed to this article.)
Again... Congratulations Julie. With your nightmare of a record and history of now confirmed corruption, I can only imagine the destruction that any product under your supervision will be able to do to the children of the world.
Un-frickin-believable... the hubris of these people.
Are there no whistle blowers that will tell the truth about what has happened from the inside? Is no one at CDC disgusted enough with this to speak up?
UPDATE:
Just got this email from Louise Kuo Habakus at Life Health Choices:
"It’ll be old home week when she joins… Eddy Bresnitz, NJ’s former state epidemiologist and deputy commissioner of health, was responsible for mandating seasonal flu and Hib vaccines before he skipped off to Merck to head up adult vaccines".
Another Update:
Someone sent me this bit of info:
Julie G. also landed on this board. With Accordia, she'll be able to get the UN and Gates Foundation to pay for Merck's products for distribution in the third world.
Of course, she will continue to tap the US Treasury to transfer American tax dollars to Merck.
September 9, 2008
Judge Rules Product Liability Suit Pre-empted by Federal Vaccine Act
Judge Rules Product Liability Suit Pre-empted by Federal Vaccine Act
Amaris Elliott-Engel
The Legal Intelligencer
September 9, 2008
A Philadelphia judge has ruled that a federal law governing the liability of pharmaceutical companies for drug vaccines pre-empts state tort claims of design defect and failure to warn in the products liability case of an 11-year-old boy who has autism.
In an apparent case of first impression, Philadelphia Common Pleas Judge Arnold L. New wrote an Aug. 27 opinion required under Pennsylvania Rule of Appellate Procedure 1925 to affirm his decision to grant summary judgment in favor of pharmaceutical defendants Aventis Pasteur Inc., Merck & Co. Inc. and Wyeth in Wright v. Aventis Pasteur.
New said both the plaintiffs' design defect and failure to warn claims were expressly pre-empted by the federal National Childhood Vaccine Injury Act. New also found that the failure to warn claim failed to raise any genuine issues of material fact that can overcome the protection the Vaccine Act provides to pharmaceutical manufacturers.
New said in his opinion that it appears no Pennsylvania state court has addressed whether §22(b) of the Vaccine Act expressly pre-empts claims of design defects against vaccine manufacturers, or whether each case has to be examined individually to determine "whether a vaccine is unavoidably safe before they gain the protection of Section 22(b)."
"Congress clearly intended when it enacted the Vaccine Act to exercise its constitutionally delegated authority to preempt all state design defect claims without case-by-case determination that the side effects are unavoidable," New wrote.
The 1986 Vaccine Act was created to provide recovery of damages to injured vaccine recipients without the requirement that the recipients prove the manufacturer was negligent and that a vaccine was defective, New said. The Vaccine Act was also aimed at preventing the undermining of national vaccine supply by expensive litigation, New said.
New noted that other courts' decisions -- including the U.S. District Court for the Eastern District of Pennsylvania's 2006 ruling in Sykes v. Glaxo-SmithKline and its 2007 ruling in Bruesewitz v. Wyeth -- have been similar.
"This court was guided by their opinions and concluded Section 22(b)(1) preempts, without there first being a case-by-case determination as to whether a vaccine is unavoidably unsafe, all state law claims that an FDA-approved vaccine was defectively designed, " New wrote.
The only contrary ruling was a 2007 Georgia state court ruling in Ferrari v. American Home Products Corp., New said.
In Wright, Jared Wright, 11, of Texas, was administered five vaccines in the first year-and-a-half of his life that contained thimerosal, a mercury-based preservative once used in vaccines to deter bacterial growth, as well as one other vaccine, New wrote. Jared's parents, Howard and Jacqueline Wright, claimed that the mercury in those six vaccines manufactured by the pharmaceutical defendants caused Jared's autism.
Plaintiffs' attorney Marc P. Weingarten of Locks Law Firm said the case is "an extremely important issue to be heard by the courts of Pennsylvania" because of the federal pre-emption issues arising in pharmaceutical and medical device litigation in both state and federal jurisdictions.
The plaintiffs argued that the defendants were negligent because the public and the medical profession were not warned about the alleged hazards of mercury in the vaccines, New said. The plaintiffs also argued that the pharmaceutical defendants failed to use ordinary cases in designing the vaccines containing thimerosal because of the risks the plaintiffs say toxic mercury poses to infants and children.
The plaintiffs said the Vaccine Act didn't automatically pre-empt the design defect claim because the vaccine defendants have the burden of proof to show on a case-by-case basis that the use of thimerosal is "‘unavoidably safe,'" New said.
Courts can interpret the Vaccine Act two ways, the plaintiffs argued, and should only interpret the Vaccine Act to pre-empt design defect claims "only if first the side effects are determined to be unavoidable on a case-by-case basis," New said.
New said Congress intended the Vaccine Act to pre-empt all state design defect claims without a case-by-case assessment if the vaccines' side effects were unavoidable because Congress didn't want instability in the vaccine market to be caused by numerous torts over vaccine injuries. That's why Congress set up its National Vaccine Injury Compensation Program, the judge said.
If the plaintiffs' argument is given credence, New said, then the protection provided by the Vaccine Act will no longer extend to vaccine manufacturers and, in turn, to the stability of the supply of child vaccines.
Manufacturers can obtain a presumption of proper warning under the Vaccine Act by providing evidence showing compliance with federal Food and Drug Administration vaccine regulations, New said. Plaintiffs can only overcome this presumption, New said, by showing the vaccine manufacturer engaged in fraud or wrongful withholding of information from the FDA regarding the vaccine prior to approval; wrongfully withheld information related to the vaccine's safety after its approval; or failed to exercise due care even though the manufacturer complied with federal laws and regulations.
Every major public health organization -- as well as the Food and Drug Administration -- that has examined the alleged link between the use of thimerosal in vaccines and neurological injury has not found a causal link, New said.
Merck defense attorney Madeline M. Sherry of Gibbons, Aventis Pasteur defense attorney Jonathan Dryer of Wilson Elser Moskowitz Edelman & Dicker and Wyeth defense attorney Reetu Dandora of Reed Smith could not be reached for comment.
July 31, 2008
The Merck Mafia
Suffolk County, New York has filed a suit against Merck and Schering-Plough with lots of big words in it like "RICO" and "indictable".
Shearlings Got Plowed/Pharmalot
July 20, 2008
Merck Clears Itself of Liability in Gardasil Injuries and Deaths
Merck is proud of Gardasil.
"Merck has analyzed the adverse events reported for GARDASIL relating to the recent reports of death and paralysis, and based on the data available to Merck, believes that no safety issue related to the vaccine has been identified."
March 26, 2008
The Gardasil Hoax
So apparently the FDA decided in 2003 that the Human Papilloma Virus was not a threat to women's long term health, that most infections resolved on their own, and HPV did not put women at much risk for cervical cancer!
Yet they went ahead and approved Gardasil for the prevention of cervical cancer, via prevention of HPV!
And now... it turns out, that women who get the vaccine may have a much higher chance of developing precancerous lesions from the vaccine than they ever had from the HPV virus!
The health officials in this country have lost their minds! Merck is going to have to change their commercials from "One Less" to "One More"
Read Mike Adam's detailed expose, complete with FDA documents and all:
UPDATE: HT to Anne Dachel for pointing out the reports of Merck's marketing of Gardasil.
March 17, 2008
Like Its Predecessor RotaShield, Offit's Rotateq May Be Linked To Intussusception
If there is such thing as a science grudge match, this qualifies, as I can't imagine that the authors of this study feel any differently about Offit than I do.
However, let's let the article speak for itself. It finds that like the last rota virus vaccine to be removed from the market, Offit's vaccine may be causing Intussusception, a nasty, life threatening disorder where the intestine actually turns inside out and starts folding in on itself like a a telescope collapses.
Rota Teq is on the CDC's vaccine schedule.
If this bears out, let's hope it is yanked even more quickly than RotaShield was.
RotaTeq vaccine adverse events and policy considerations
David A. Geier, Paul G. King, Lisa K. Sykes, Mark R. Geier
The Institute of Chronic Illnesses, Inc., CoMeD, Inc., The Genetic Centers of America,
Potential conflicts of interest: David A. Geier has been a consultant in vaccine/biologic cases before the no-fault National Vaccine Injury Compensation Program (NVICP) and in civil litigation. Mark R. Geier has been a consultant and expert witness in vaccine/biologic cases before the no-fault NVICP and in civil litigation. Paul G. King and Lisa K. Sykes have no conflicts of interest.
Background: Rotavirus is the leading cause of severe gastroenteritis in children <5 years-old worldwide. On February 3, 2006, the US Food and Drug Administration licensed RotaTeq™ (Merck and Co.), a bioengineered combination of five human-bovine hybridized reassortment rotaviruses. In August of 2006, the Advisory Committee on Immunization Practices recommended RotaTeq for routine vaccination of US infants administered orally at the ages 2, 4, and 6 months. Material/Methods: An evaluation of data reported to VAERS following the fi rst fi ve quarters of post-marketing surveillance of RotaTeq was undertaken. Trends in adverse events reported following RotaTeq and cost effectiveness calculations of RotaTeq in the context of the disease burden of rotavirus in the US were examined. Results: From February 3, 2006 through July 31, 2007, a total of 160 (of the 165 reported) intussusception and 11 (of the 16 reported) Kawasaki disease adverse event reports were identifi ed when RotaTeq was administered or co-administered with other vaccines. Time-trend analyses showed that there were signifi cant increases in the total number of intussusception and Kawasaki disease adverse events entered into VAERS in comparison to previous years. Conclusions: These observations, coupled with limited rotavirus disease burden, cost-effectiveness, and potential contact viral transmission concerns, raise serious questions regarding the use of RotaTeq in the US. Healthcare providers should diligently report adverse events following RotaTeq vaccination to VAERS, and those who have experienced a vaccine-associated adverse event should be made aware that they may be eligible for compensation from the no-fault National Vaccine Injury Compensation Program (NVICP). key words: gastroenteritis • gastrointestinal hemorrhage • rotavirus infection • vaccine adverse event reporting system
March 14, 2008
MMR + Chicken Pox Vaccine = More Seizures
"It found a rate of febrile seizure of nine per 10,000 vaccinations among MMRV recipients, and four per 10,000 among children who got separate MMR and chicken pox shots. Of 166 children who had febrile seizures after either type of vaccination, 26 were hospitalized and none died, the CDC said."
So CDC removes its preference for the MMRV vaccine, but does not change it's preference to the MMR and separate chicken pox vaccine. It just does not state a preference. Because half the seizures is not worth stating a preference?!
This does not just call for a preference, it seems to make the MMRV obsolete. What they had before the MMRV was safer.
Is the CDC's priority the health of children or the health of Merck's bottom line?
WASHINGTON (Reuters) - Children who get a combined vaccine against measles, mumps, rubella and chicken pox are slightly more likely to have seizures compared to those getting two separate shots for the same diseases, U.S. officials said on Thursday.
The seizures are not usually life-threatening and the U.S. Centers for Disease Control and Prevention said it was no longer expressing a preference that children get the so-called MMRV combined vaccine rather than two shots -- the MMR vaccine against measles, mumps and rubella (German measles) and a separate one against varicella (chicken pox).
The CDC said it made the change after seeing evidence that children who got the combined MMRV vaccine faced an elevated, but still very small, risk of suffering febrile seizures after vaccination compared to those who got the two shots.
A febrile seizure is a convulsion in young children associated with an increase in body temperature, often from an infection. While frightening, the seizures are not usually dangerous and only a small percentage of children who experience one go on to develop epilepsy.
Dr. John Iskander, the acting director of the CDC's Immunization Safety Office, said it remained very important that parents get their children vaccinated against these diseases.
"These are vaccines that have had enormous public health benefits," Iskander said.
The CDC said the availability of the MMRV vaccine, made by pharmaceutical company Merck, already was limited in the United States because of manufacturing constraints unrelated to vaccine safety, and was not expected to be widely available until 2009.
The CDC said a study examined the risk for febrile seizures seven to 10 days after vaccination among 43,353 children ages 12 months to 23 months who received the MMRV vaccine and 314,599 children of the same age who received the MMR vaccine and chicken pox vaccine administered separately.
It found a rate of febrile seizure of nine per 10,000 vaccinations among MMRV recipients, and four per 10,000 among children who got separate MMR and chicken pox shots. Of 166 children who had febrile seizures after either type of vaccination, 26 were hospitalized and none died, the CDC said.
July 9, 2007
NAA: No on California AB 16
California legislators need to hear from ALL of us on AB 16 BEFORE July 11!
California’s AB 16, approved last week by the Senate Education Committee, would remove all public input and legislative review on childhood vaccines added to the mandatory immunization schedule, automatically adding every new vaccine approved by the CDC's Advisory Committee on Immunization Practices (ACIP).
- Passage of this bill will set a dangerous precedent for all of us, whether or not we live in California.
- We’ve seen what public input has done to prevent Gardasil from becoming mandated in some states, as even many in the mainstream medical community opposed forcing the HPV shot on young girls.
- Merck, the drug company behind AB 16 and Gardasil, wants to ensure that public opinion, scientific debate, and common sense will never again stand in the way of profit.
- If this bill passes in California, similar legislation will most likely find its way to every other state.
PLEASE ACT NOW!
The California Senate Health Committee meets this Wednesday, July 11.
PLEASE CALL, FAX, OR EMAIL EACH MEMBER OF THE COMMITTEE LISTED BELOW WITH LINKS TO CONTACT INFORMATION, AND TELL THEM TO VOTE NO ON AB 16:
Senator Sheila Kuehl (Chair)
Senator Samuel Aanestad (Vice Chair)
Senator Elaine Alquist
Senator Gilbert Cedillo
Senator Dave Cox
Senator Abel Maldonado
Senator Gloria Negrete McLeod
Senator Mark Ridley-Thomas
Senator Darrell Steinberg
Senator Mark Wyland
Senator Leland YeeLast week’s testimony from parent Rick Rollens is below. Please read this powerful statement, contact the California Senate Health Committee members now, and pass this message along to family members and friends. We can’t let Merck get away with stealing our civil rights, gaining even more power to foist poorly tested and potentially harmful products on our children.
Mr. Chairman and Members:
My name is Rick Rollens. This is my 33 year of being in and around the Capitol. For 24 years I served in the State Senate in numerous positions including a chief of staff to a Senator, chief consultant to the Senate Rules Committee, creator and director of the Office of Senate Floor Analyses, and finally as Secretary of the Senate. In 1996 I resigned my post ion as Secretary of the Senate in order to dedicate my life to finding effective treatments and a cure for my beloved son Russell who suffers from vaccine induced regressive autism.Since leaving the Senate, I have been extremely active in the autism world. I am a co-founder of FEAT...Families for Early Autism Treatment, a co-founder of the UC Davis M.I.N.D. Institute, a Speaker's appointee to the Legislative Blue Ribbon Commission on Autism, Superintendent O'Connell's appointee to his Autism Advisory Committee, I have served as a national board member of ASA, the NIH Autism Advisory Committee, and currently serve on numerous autism organizations throughout California, the nation, and the world. My family and I have been featured in dozens of local, state, national and international media stories about autism and the autism epidemic, the crown jewel of them all is this (SHOW NEWSWEEK) cover story in Newsweek magazine that featured my son Russell on the cover and a feature on Russell's story of his decent into autism at 6 months old after receiving numerous shots at his well baby check up and immediately suffering a classic adverse vaccine reaction leading to his acquired full syndrome autism. That day changed his life and the lives of ALL who know and love him. Russell is not alone.
Today, California is adding 10 new children a day, seven days a week, like Russell to our DD system. In 1980 when California first enacted it's mandatory immunization law, autism accounted for 3% of all the intakes into our DD system. Today, autism only the fastest growing condition entering the system but now accounts for 64% of all the new intakes. In 1980 the incidence of autism was 1 in 10,000, today it is 1-150, and in some areas high as 1-84 children. Twenty years ago there were 2700 persons with autism in our system, today there are 34,000. In the past 9 months alone, we added more children with autism to our system then we did over the 16 YEAR period from 1971-1987! 886 new children in the past 3 months alone.
The most telling fact is that over 91%, or 9 out of 10 persons currently in our system were born after 1980, the year that California's mandatory immunization law was enacted. There is a tsunami of young children aged 3 to 17 years old accounting for nearly 80% of the autism population moving through the system.
I am here today to vehemently oppose AB 16. AB 16 represents an outrageous and arrogant attempt by the makers of the HPV vaccine and Vioxx, as well as those who front for them in the public health community, to strip away from the Legislature and the Governor the responsibility that has been in statute for nearly 30 years to review and approve or reject the addition of new vaccines to the mandatory childhood immunization schedule; and instead, turn over that responsibility to one and a group of their own, a non-accountable bureaucrat, the state Director of Public Health and a Committee 3,000 miles away of vaccine promoters who have yet to reject an application for adding a new vaccine to the schedule, and numerous members of which are personally and professionally conflicted for accepting research and professional funding and career opportunities from the same vaccine manufactures that are suppose to be regulating. Their behavior and actions have become subject to Congressional investigation and review.
AB 16 as introduced would have added Merck's HPV vaccine to the mandatory schedule. After extensive public hearing and debate in the Assembly Health Committee, it was clear that there was little support to approve the bill and the author refused to even let the bill come up for a vote. This was the second new vaccine that has been rejected by the Legislature in the past five years. I guess enough was enough in the minds of the vaccine manufacturers and their followers. The bill was subsequently gutted and amended the bill to include the provisions before you today.
Keep in mind, that today in California children receive up to 30 doses of vaccines by the age of 6 years old, most of which are administered starting moments after birth through the first two years of life when healthy brain development is most important. If the provisions of AB 16 had been in effect during this current decade, the number of doses of vaccines our children would have been subjected to would have increased to 40 doses. Throughout the country, including right here at the M.I.N.D. Institute, dozens of research projects are currently underway examining the connection between the immune system, vaccines, and autism.
And lastly, be aware that there are over 300 new vaccines currently in development and in the pipeline, including vaccines for such things as nicotine addiction, diarrhea, mononucleosis, cocaine, methamphetamine, and stomach ulcers. These vaccines, as well as vaccines currently in use today contain such potent toxic and poisonous agents as mercury, aluminum, formaldehyde, aborted fetal tissue, MSG, live viruses, and killed bacteria.
Mr. Chairman and Members, the system we have in place today has served us well for nearly three decades. You and your constituents and future members of the legislature and their constituents have a real say in the very serious issue of what new vaccines are added to MANDATORY childhood immunization schedule. There is sunshine in the current process, this bill takes away the sunshine away and replaces it with a wink and a nod by unaccountable bureaucrats and members of a Committee that have not seen a vaccine they can say no to.
On behalf of the children and their families of today, and the children yet to be born, please reject this horrific proposal. Keep this process in the hands of the people's representatives, do not hand over the future of our children's very health to those who would profit both personally and professionally by approving this bill. Please vote no. Thank you.
June 27, 2007
Offit's RotaTeq Safety Labeling Updated to Include Cases of Kawasaki Disease
RotaTeq Safety Labeling Updated to Include Cases of Kawasaki Disease
http://www.medscape.com/viewarticle/558530?src=mp
Yael Waknine
Medscape Medical News 2007. © 2007 Medscape
June 19, 2007 — Changes have been made to the adverse reactions and postmarketing sections of the safety labeling for a live, oral, pentavalent rotavirus vaccine (RotaTeq, Merck and Company, Inc); it now includes cases of Kawasaki disease, the US Food and Drug Administration (FDA) told healthcare professionals on Friday.
The poorly understood disease is uncommon in children, is characterized by high fever and blood vessel inflammation, and affects the lymph nodes, skin, mouth, and heart.
During a phase 3 clinical trial, 5 cases of Kawasaki disease were reported among 36,150 infants who received the vaccine, compared with only 1 case among 35,536 who were given placebo, according to an alert sent from MedWatch, the FDA's safety information and adverse event reporting program.
Three other cases have been reported through the Vaccine Adverse Event Reporting System (VAERS) since the vaccine was approved on February 3, 2006. There is no known cause-and-effect relationship between the use of this or any other vaccine and Kawasaki disease, the FDA said, noting that the cases reported to date are not more frequent than what would be expected to occur by coincidence.
Rotavirus vaccine is indicated for the prevention of rotavirus gastroenteritis in infants and children, which is caused by the serotypes G1, G2, G3, and G4, when administered orally as a 3-dose series to infants between the ages of 6 and 32 weeks.
Healthcare professionals are encouraged to report cases of Kawasaki disease and other adverse events potentially associated with the vaccine to VAERS by going online at www.vaers.hhs.gov or calling 1-800-822-7967 for a report form.
Additional information about the use of the vaccine can be obtained by contacting the FDA’s Center for Biologics Evaluation and Research at 1-800-835-4709 or by e-mail at octma@cber.fda.gov.
